Biomedical engineers at UC Davis have come up with a new tool for tracing interactions between proteins. The new, light-activated tool could have wide applications in cell biology.
A key problem in cell biology is figuring out which proteins interact with each other and when. A useful tool for this is proximity labeling: the protein of interest, or bait, is tagged with an enzyme that will label nearby proteins (the ‘prey’) with the small molecule biotin. Proteins labeled with biotin can then be detected by a variety of means.
This is rather like coating a football with fluorescent paint and then seeing which players pick up the ball by the paint on their hands.
But there are some problems with the existing proximity labeling technology, said Sanjeevi Sivasankar, professor of biomedical engineering at UC Davis.
“You can’t turn the labeling on or off, so you can quickly end up with thousands of labeled partners,” he said. Think of that football going into the crowd and getting passed around thousands of spectators.
Sivasankar’s lab studies cadherins, a class of proteins that stick cells together. Cadherins are important for maintaining structure of tissues. By tightening or loosening the connections between cells, they may hold cancer cells in place or allow them to spread.
Light Activated BioID
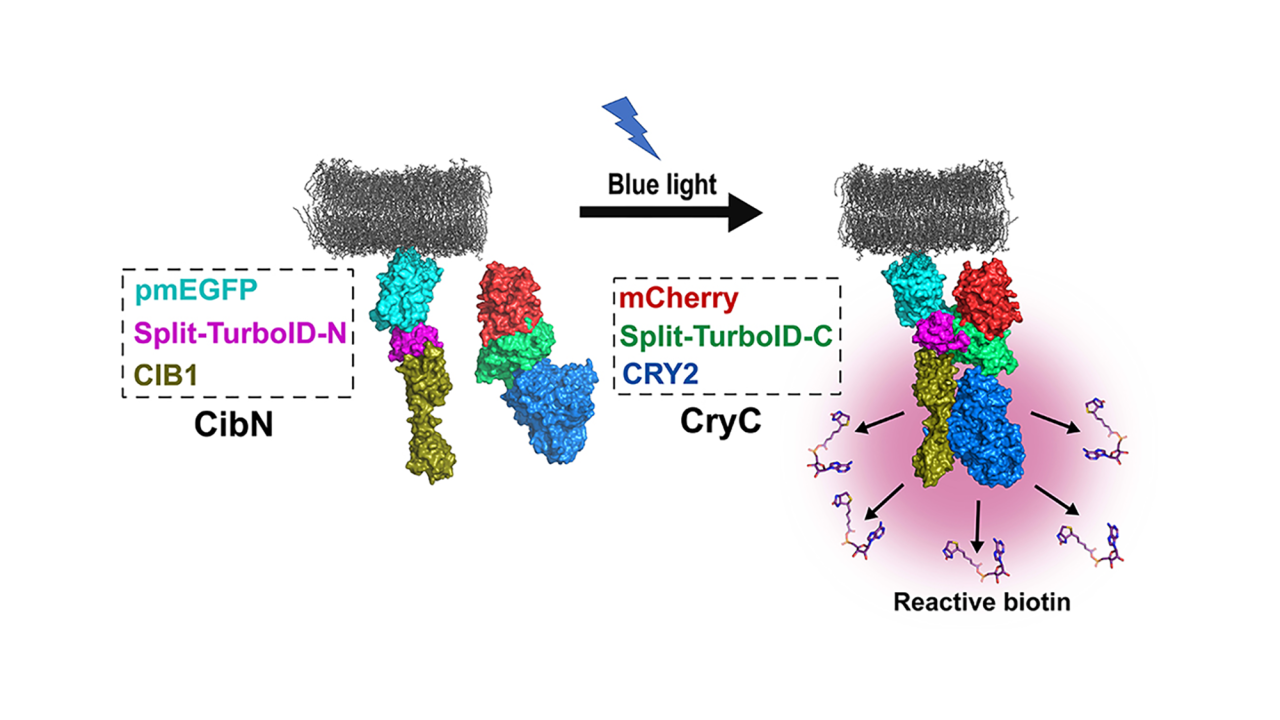
Omer Shafraz, postdoctoral scholar in Sivasankar’s lab and graduate student Carolyn Davis came up with a new method. They made use of the fact that the enzyme that carries out biotin labeling, TurboID, can be split into halves that cannot work independently.
The team combined the halves with another pair of proteins that forms dimers (pairs up with each other) when exposed to light. Shining light on the new molecules brings the halves together and starts biotin labeling: when the light is turned off, the halves fall apart and labeling stops.
The new approach, which the team call Light Activated BioID or LAB, makes it possible to label proteins that interact with a target in a short time window, reducing the number of false positives.

First Person: Carolyn Davis
Coauthor Carolyn Davis discusses the work in this Q&A for the Journal of Cell Science.
“We think this will be of very broad use to the cell biology community,” Sivasankar said. “The key to a lot of biology is figuring out which proteins are binding to each other, so there are potentially broad applications in biology generally.”
Before joining the UC Davis Department of Biomedical Engineering in 2018, Sivasankar’s work had focused on building hardware such as microscopes and other equipment. The collaborative environment at UC Davis had enabled him to incorporate more biology into his work, he said.
“Now I’m building wetware, a protein that can be turned on or off with light,” he said.
The work is published Oct. 11 in the Journal of Cell Science and was supported in part by grants from NIH and NSF.
Media Resources
Light-activated BioID – an optically activated proximity labeling system to study protein–protein interactions (Journal of Cell Science)
First Person: Carolyn Davis (Journal of Cell Science)